Dr Christen Rune Stensvold is a Senior Scientist and Public Health Microbiologist with specialty in parasitology. He has a Bachelor degree in Medical Sciences, an MSc in Parasitology, and a PhD in Health Sciences. He has been based at Statens Serum Institut, Copenhagen, since 2004. Since 2006, he has authored/co-authored more than 80 articles in international, peer-reviewed scientific journals. In 2013, he was awarded the Fritz Kauffmann Prize for his contribution to clinical microbiology in Denmark. For many years, he has been pursuing the role of common intestinal micro-eukaryotes in human health and disease. Follow Rune on Twitter @Eukaryotes.
Blog
Non-alcoholic fatty liver disease and diabetes—are they symptoms of gut dysbiosis?
August 18, 2016 | Christen Rune Stensvold
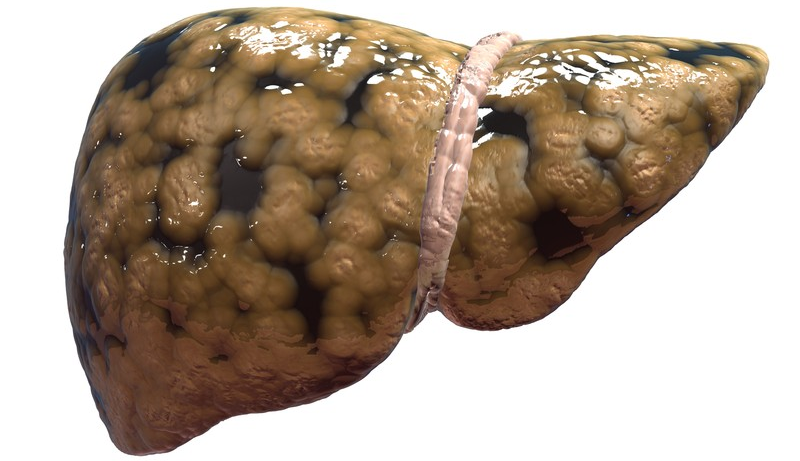
The idea that obesity is a direct consequence of changes in gut microbiota structure and function that lead to enhanced extraction of energy from the host diet has been discussed for some years. Studies now suggest that associated diseases such as non-alcoholic fatty liver disease (NAFLD) and diabetes may also be considered consequences of intestinal dysbiosis.
A leading cause of liver transplantation, NAFLD may now top the list of the most common liver diseases in developed countries. In an article published in Gastroenterology earlier this year, Betrapally and colleagues argue that “…the development and progression of fatty liver, alcoholic fatty liver disease, and NAFLD all appear to be influenced by the composition of the [gut] microbiota.”1 The way the gut microbiota influences the progression of these diseases appears to be complex, potentially involving diet-induced changes in bacterial metabolomes and microbial interactions with, for instance, bile acids and gut hormones such as glucagon-like peptide 1 (GLP1).1,2 An incretin secreted by enteroendocrine L cells, GLP1 induces pancreatic β-cell proliferation, maintains glucose-dependent insulin secretion and inhibits glucagon release, gastric emptying and food intake. Short-chain fatty acids (SCFAs) synthesized by certain bacteria activate the G-protein-coupled receptors GPR41 and GPR43, promoting secretion of GLP1.1
This was exemplified in a randomized controlled trial (RCT), in which Alisi and colleagues investigated the effect of the probiotic VSL#3, a mixture of eight probiotic strains (Streptococcus thermophilus, bifidobacteria [B. breve, B. infantis, B. longum], Lactobacillus acidophilus, L. plantarum, L. paracasei, and L. delbrueckii subsp. bulgaricus) in children with NAFLD, using changes in the severity of the fatty liver disease as the primary outcome.2 They found that a 4-month supplement of VSL#3 significantly improved NAFLD, probably through VSL#3-dependent reversal of dysbiosis. Of note, however, is the fact that no data were included on gut microbiota profiling prior to or after intervention. Nevertheless, the authors speculated that restoration of normal gut flora led to reduced intestinal permeability, increased production of SCFAs and anorexogenic gut hormones, including GLP1, as well as enhanced insulin sensitivity.
The effect of consuming 300g of probiotic yoghurt containing Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 every day for 8 weeks on selected metabolic parameters was studied in an RCT comprising 72 NAFLD patients.3 Patients consuming the probiotic yoghurt exhibited a reduction in the levels of serum hepatic enzymes (alanine aminotransferase and aspartate aminotransferase), serum total cholesterol, and low-density lipoprotein cholesterol. Again, no microbiota profiling was performed in order to try to link the findings with potential restoration of gut eubiosis.
Meanwhile, other studies—although not intervention studies—have taken to investigating the gut microbiota profiles of patients with NAFLD lesions such as non-alcoholic steatohepatosis and fibrosis. These studies have identified independent associations between the predominance of certain bacterial groups and the presence of fatty liver disease.4,5 The fact remains, however, that we still need intervention studies that include pre-intervention and post-intervention gut microbiota profiling, preferably including data on the gut metabolome as well. And, as pointed out by Wieland and colleagues,6 we still need data in order to be able to delineate causality and obtain a mechanistic understanding of how in fact obesity and related metabolic and hepatic disease might reflect changes in gut microbiota structure and function.
We still need intervention studies that include pre-intervention and post-intervention gut microbiota profiling
On a different—yet related—note, a study has just been published in Nature identifying Prevotella copri as a driver of insulin resistance and potentially the development of type-2 diabetes.7 Mice fed P. copri developed increased serum levels of branched-chain amino acids, insulin resistance and reduced glucose tolerance. Hence, P. copri was identified as one of the bacterial species most critical to the development of insulin resistance. This finding suggests that type-2 diabetes is effectively the result of intestinal dysbiosis with predominance of certain bacteria such as P. copri.
The results of future research will reveal the extent to which obesity and associated metabolic and hepatic diseases may be alleviated and perhaps even treated via gut microbiota manipulation with prebiotics, probiotics, synbiotics, antibiotics or other compounds such as SCFAs.8
If you are interested in the role of the gut microbiota in the development of NAFLD, especially changes in SCFA metabolism, I highly recommend perusing the review by Leung and colleagues in Nature Reviews Gastroenterology & Hepatology.9 For even more information on microbiota-associated pathogenesis of liver disease and its complications, I suggest reading the reviews by Schnabel and Brenner,10 Boursier and Diehl,11 Quigley and Monsour,12 and Abdou and colleagues.13 To find out more about the use of probiotics in the therapy of NAFLD, the reviews by Putignani et al.14 and Tarantino and Finelli15 are also worth consulting.
References
- Betrapally NS, Gillevet PM and Bajaj JS. Changes in the intestinal microbioma and alcoholic and nonalcoholic liver diseases: Causes of effects? Gastroenterology 2016; 150: 1745–1755. http://www.gastrojournal.org/article/S0016-5085(16)00294-8/abstract
- Alisi A, Bedogni G, Beviera G, et al. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2014; 39: 1276–1285. http://onlinelibrary.wiley.com/doi/10.1111/apt.12758/abstract
- Nabavi S, Rafraf M, Somi MH, et al. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci 2014; 97: 7386–7393. http://www.journalofdairyscience.org/article/S0022-0302(14)00701-2/abstract
- Boursier J, Mueller O, Barret M, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016; 63: 764–775. http://onlinelibrary.wiley.com/doi/10.1002/hep.28356/abstract
- Jiang W, Wu N, Wang X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep 2015; 5: 8096. http://www.nature.com/articles/srep08096
- Wieland A, Frank DN, Harnke B, et al. Systematic review: microbial dysbiosis and non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2015; 42: 1051–1063. http://onlinelibrary.wiley.com/doi/10.1111/apt.13376/abstract
- Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact hos serum metabolome and insulin sensitivity. Nature 2016; 535: 376–381. http://www.nature.com/nature/journal/v535/n7612/abs/nature18646.html
- Jin CJ, Sellmann C, Engstler AJ, et al. Supplementation of sodium butyrate protects mice from the development of non-alcoholic steatohepatitis (NASH). Br J Nutr 2015; 114: 1745–1755. http://journals.cambridge.org/action/displayAbstract?fromPage=online&aid=10029322&fileId=S0007114515003621
- Leung C, Rivera L, Furness JB, et al. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol 2016; 13: 412–425. http://www.nature.com/nrgastro/journal/v13/n7/full/nrgastro.2016.85.html
- Schnabl B and Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014; 146: 1513–1524. http://www.gastrojournal.org/article/S0016-5085(14)00077-8/abstract
- Boursier J and Diehl AM. Implication of gut microbiota in nonalcoholic fatty liver disease. PLoS Pathog 2015; 11: e1004559. http://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1004559
- Quigley EM and Monsour HP. The gut microbiota and nonalcoholic fatty liver disease. Semin Liver Dis 2015; 35: 262–269. https://www.thieme-connect.com/DOI/DOI?10.1055/s-0035-1562946
- Abdou RM, Zhu L, Baker RD, et al. Gut microbiota of nonalcoholic fatty liver disease. Dig Dis Sci 2016; 61: 1268–1281. http://link.springer.com/article/10.1007/s10620-016-4045-1
- Putignani L, Alisi A and Nobili V. Pediatric NAFLD: the future role of patient-tailored probiotics therapy. J Pediatr Gastroenterol Nutr 2016; 63 Suppl 1: S6–8. http://journals.lww.com/jpgn/Fulltext/2016/07001/Pediatric_NAFLD___the_Future_role_of.3.aspx
- Tarantino G and Finelli C. Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Future Microbiol 2015; 10: 889–902. http://www.futuremedicine.com/doi/abs/10.2217/fmb.15.13?journalCode=fmb
-
About the Author





Please log in with your myUEG account to post comments.