WHAT IS YOUR CLINICAL DIAGNOSIS AT THIS POINT?
a) Biliary colic
b) Acute cholecystitis
c) Choledocholithiasis
d) Cholangitis
e) Acalculous cholecystitis
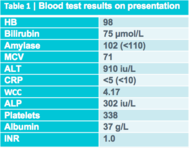
A 33-year-old Chinese woman presented to our hospital acutely with increasing jaundice and pruritus. She had been experiencing upper abdominal pain for 2 months and had been jaundiced for about 4 weeks. Some weight loss and sweats had also occurred. On examination, she was apyrexial, jaundiced and slightly tender in the epigastrium. Her blood test results are shown in table 1.
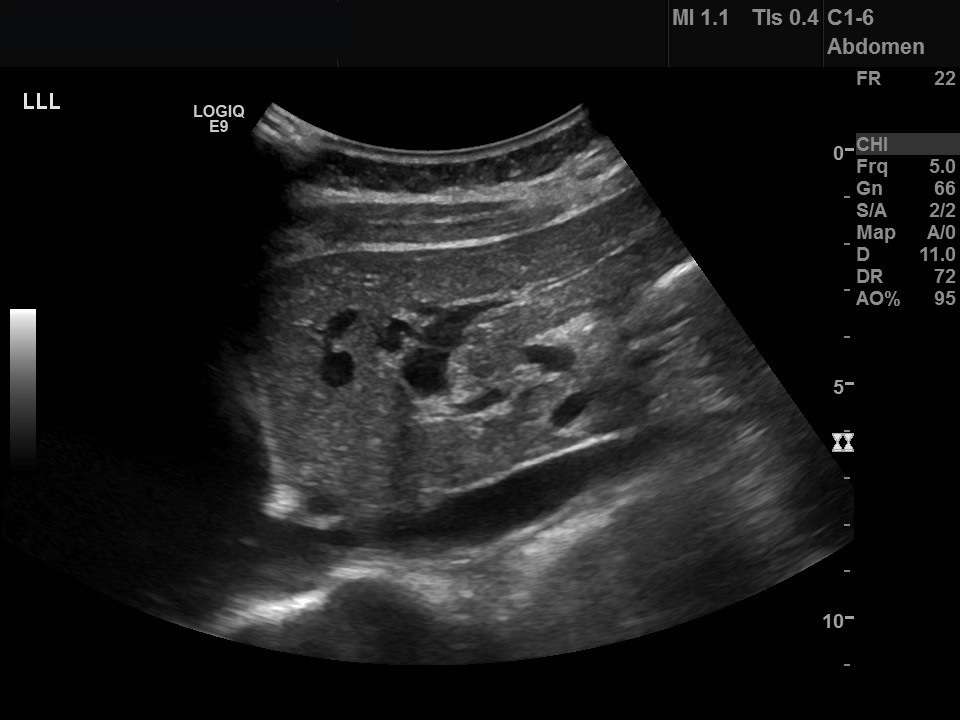
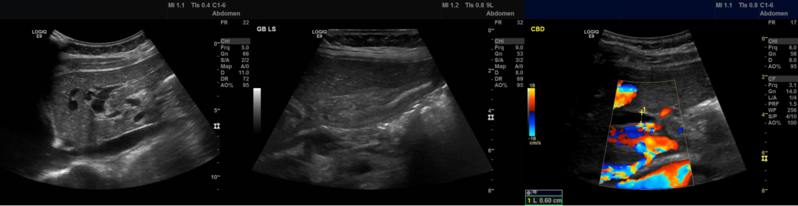
An abdominal ultrasound was organised and the images of the left liver lobe, gallbladder in longitudinal section and the common bile duct are shown in figure 1. The ultrasound was reported as, “The gallbladder is extremely thick walled and hyperaemic, but non tender on scanning. Intrahepatic biliary duct dilatation noted, but the CBD [common bile duct] is normal calibre at the porta and 6 mm in AP [antiposterior] diameter. No stones identified. The pancreas and pancreatic duct appear normal. The liver, abdominal aorta, spleen and kidneys appear normal. No ascites seen.”
a) Biliary colic
b) Acute cholecystitis
c) Choledocholithiasis
d) Cholangitis
e) Acalculous cholecystitis
Correct answer: c.
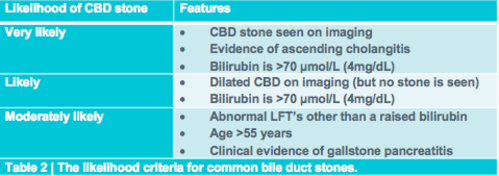
Choledocholithiasis should be your clinical diagnosis at this point. This is because the ‘strong likelihood criteria’ are fulfilled as follows. First, although no stone is visible on imaging, the CBD is dilated. Second, the bilirubin level is elevated above 4mg/dL (70 µmol/L). In this case, there is no clinical evidence of an ascending cholangitis (fever, jaundice, ↑bilirubin, ↑WCC and a dilated CBD on ultrasound) and for this reason the likelihood of choledocholithiasis is strong rather than very strong (table 2).
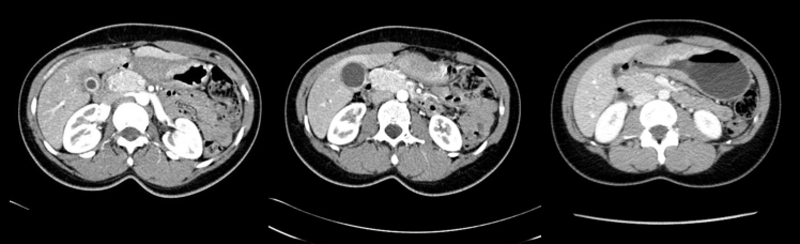
To search for further gallstones a CT scan was subsequently requested (figure 2). The scan was reported as follows: “The gallbladder is inflamed and there may be stones in the fundus of the gallbladder, and I suspect there is also one stone obstructing the neck of the gallbladder. This is causing extrinsic compression of the bile duct at the porta hepatis with intrahepatic ductal dilation. The distal bile duct is collapsed. Normal appearance of the pancreas, kidneys, adrenal glands and spleen.”
a) Type I Mirizzi’s syndrome
b) Type II Mirizzi’s syndrome
c) Type III Mirizzi’s syndrome
d) Type IV Mirizzi’s syndrome
Correct answer: a.
The CT report essentially describes Mirizzi syndrome type 1. Pablo Mirizzi was an Argentinian surgeon who first described the obstruction of the common hepatic duct (CHD) by an impacted stone in the cystic duct or in the neck of the gallbladder (Hartmann's pouch). In Mirizzi syndrome type I there is no fistula between the gallbladder and CHD. By contrast, Mirizzi syndrome type II, III and IV all have a fistulous communication. Of course it is difficult to tell on imaging if there is a fistula. For this reason, the type of Mirizzi syndrome present is usually something that’s discovered during surgery.
a) Intravenous antibiotics and cholecystectomy within 2 months
b) Endoscopic retrograde cholangiopancreatography (ERCP) and stent placement, followed by cholecystectomy within 2 weeks
c) Intravenous antibiotics and cholecystectomy within 2 days
Correct answer: c.
In the past, the preference was for delaying cholecystectomy in the setting of acute cholecystitis until resolution of acute inflammation. However, several studies demonstrated the superiority of early surgery and it was subsequently endorsed in a Cochrane review.1 Compared with delayed cholecystectomy (>6 weeks after onset of symptoms), early laparoscopic cholecystectomy (with definitions ranging from 24 hours to <7 days after the onset of symptoms) is associated with decreased length of hospital stay and no difference in complications or conversion to open cholecystectomy.
The standard of care for patients at high risk of choledocholithiasis is laparoscopic cholecystectomy with intraoperative cholangiography during the index admission. For those patients who undergo ERCP as primary management for choledocholithiasis, cholecystectomy is still required during the same admission. Delayed cholecystectomy is associated with an increased risk of further pain, readmission with acute cholecystitis or biliary pancreatitis (36% versus 2% at 6 weeks).2 Furthermore, there is no increased risk of cystic stump leakage, bleeding, wound infection or abscess formation in patients who undergo early surgery.
In this case, the patient did undergo laparoscopic cholecystectomy, but to the surgeon’s surprise there were no stones within the thickened gallbladder or within the common bile duct.
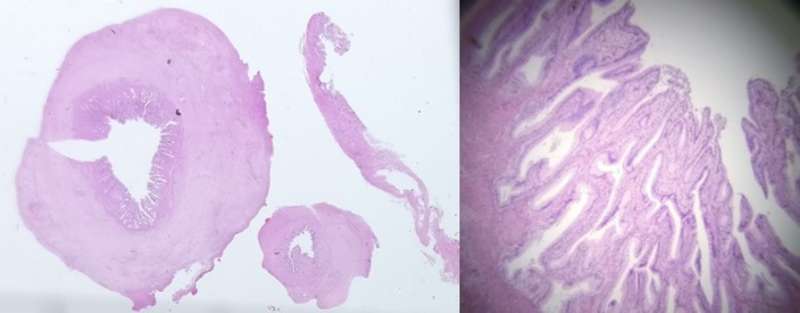
The histology of the gallbladder (figure3) was reported as:
“Gallbladder measures 52 x 30 x 20 mm. The serosa is congested and wall thickness is 3 mm. No stone and no focal lesions. There is subacute cholecystitis with myofibroblastic proliferation of the wall and mild acute-on-chronic inflammation. There is no mucosal dysplasia.”
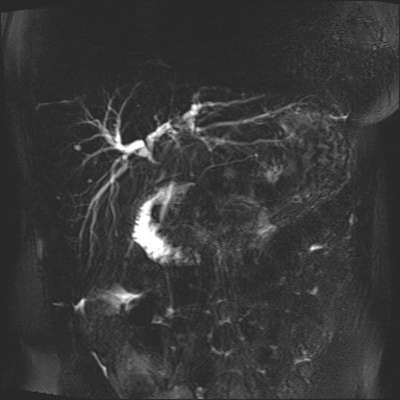
The jaundice did not resolve after surgery and for this reason an MRI scan was carried out. The MRI scan confirmed there was a stricture at the CHD with mild intrahepatic ductal dilatation. The distal bile duct was collapsed (figure 4).
At this point, we decided to carry out an ERCP to sample the stricture and place a stent (video 1).
Video 1 | ERCP and EUS of the case patient.
a) Primary cancer of the gallbladder
b) Cholangiocarcinoma
c) The CBD had been clipped by mistake
d) Something else
Correct answer: d.
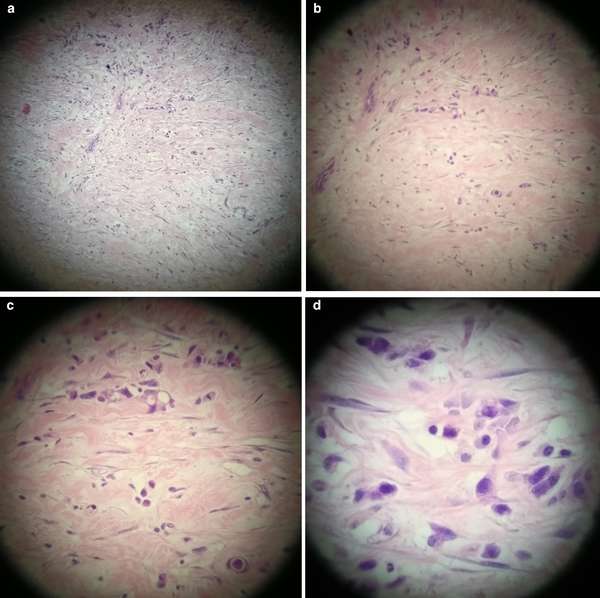
The gallbladder was as thickened, as would be expected in a case of cholecystitis, but there was only mild inflammation of the gallbladder mucosa. This doesn't make any sense! Of course, in an acute cholecystitis there should be an INTENSE inflammation of the mucosa. A second look at the thickened gallbladder wall (figure 5) led to a revelation.
“There is poorly differentiated diffuse type adenocarcinoma in the fibrotic wall with single and files of small neoplastic epithelial cells. The tumour appears to be coming from outside the gallbladder as there is no involvement of the gallbladder mucosa. The differential diagnosis will include [a] gastric primary [adenocarcinoma] or, less likely a lobular breast carcinoma. Immunohistochemistry shows this diffusely infiltrating adenocarcinoma is positive for AE1/3, CK7 [and] CDX2. It is negative for ER, PR, TTF1, CK20 [and] CA125. The immuno profile, together with the diffuse pattern of infiltration, both point to a primary gastric adenocarcinoma of diffuse type.”
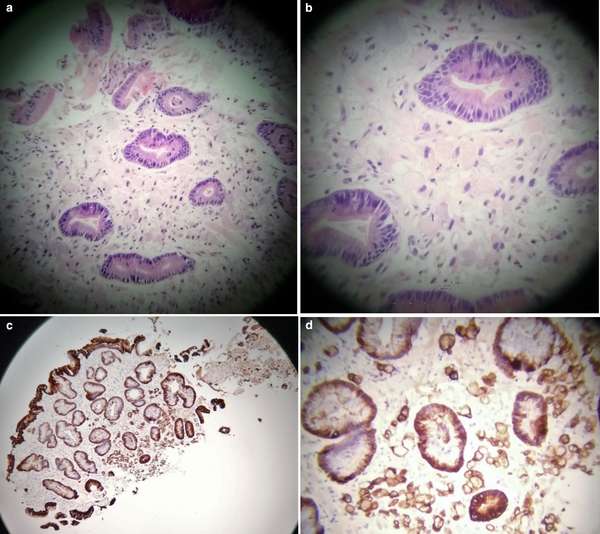
At about the same time the findings from the gastric antral biopsy samples (figure 6) were reported.
“Fragments of gastric antral type mucosa with background features of reactive gastritis. In some of the fragments, there is infiltration of the lamina propria by individual neoplastic cells with intracellular mucin and signet ring morphology consistent with poorly differentiated, diffuse type gastric adenocarcinoma consistent with linitis plastica. The features are similar to the histology of the gallbladder resection previously reported.”
Of course, this patient has T4 stage disease. At this stage, only one question remained: is there any evidence of an E-cadherin mutation? Somewhat surprisingly this proved not be the case. Immunohistochemistry testing revealed E-cadherin in both normal and malignant cells. Subsequently, the MDT decision was to offer palliative chemotherapy with avelumab, an investigational (JAVELIN Gastric 300, phase 3 trial; NCT02625623), fully human anti-PD-L1 IgG1 monoclonal antibody. Avelumab binds and blocks a receptor that switches off CD8+ T-cell-mediated programmed cell death. In other words, avelumab is an immune checkpoint blockade cancer therapy that aims to stop the stimulation of the PD-1/PD-L1 receptor/ligand complex and increase the CD8+ T-cell immune response.
There are several take-home messages from this case:
Bjorn Rembacken is at Leeds Teaching Hospitals NHS Trust, Leeds, UK. He was born in Sweden and qualified from Leicester University in 1987. He undertook his postgraduate education in Leicester and in Leeds. His MD was dedicated to inflammatory bowel disease. Dr Rembacken was appointed Consultant Gastroenterologist, Honorary Lecturer at Leeds University and Endoscopy Training Lead in 2005. Follow Bjorn on Twitter @Bjorn_Rembacken
Please log in with your myUEG account to post comments.