Jacques Bergman is a Professor of Gastrointestinal Endoscopy and Head of Endoscopy in the Department of Gastroenterology and Hepatology at Amsterdam UMC, Roos Pouw, is Principle Investigator and Associate Professor at the same department. They both lead the oesophageal research team, which has a longstanding interest in endoscopic imaging and treatment of Barrett’s oesophagus. Eva Verheij is a PhD fellow in the group and her focus is on the endoscopic management of Barrett neoplasia.

Barrett’s oesophagus is a premalignant condition of the distal oesophagus predisposing to oesophageal adenocarcinoma. Given the potential for malignant progression and the poor prognosis of eosophageal adenocarcinoma when diagnosed at a symptomatic stage, patients with known Barrett oesophagus undergo regular endoscopic surveillance to detect neoplastic progression at an early and preferably endoscopically, treatable stage. Endoscopic management of early Barrett oesophagus neoplasia consists of a combination of endoscopic imaging, endoscopic resection and endoscopic ablation. Below we discuss a number of mistakes that are frequently made when managing Barrett oesophagus neoplasia and how to avoid them. Much of this discussion draws on existing guidelines (for background reading, check the ESGE Barrett oesophagus guideline1), but in many instances the underlying evidence (even in the guideline) is missing and therefore many of our practically driven recommendations are based on common sense and our experience in this field.
© UEG 2021 Verheij, Pouw and Bergman.
Cite this article as: Verheij EPD, Pouw RE and Bergman JJ. Mistakes in endoscopic treatment of Barrett oesophagus neoplasia and how to avoid them. UEG Education 2021; 21: 35–39.
Eva P.D. Verheij is a PhD fellow, Roos E. Pouw is Principal Investigator and Associate Professor, and Jacques J. Bergman is a Professor of Gastrointestinal Endoscopy and Head of Endoscopy, Department of Gastroenterology and Hepatology, Amsterdam University Medical Centers, location Free University Center, Amsterdam, the Netherlands.
Images: courtesy of J.J. Bergman and BEST Academia.
Illustrations: J. Shadwell.
Correspondence to: [email protected]
Conflict of interest: The authors declare there are no conflicts of interest in relation to this article.
Published online: December 20, 2021.
With inadequate cleaning and immediately ‘jumping’ to obtain the inevitable random biopsy samples there is no chance that you will detect the more subtle Barrett lesions. So, please spend a couple of minutes to optimize imaging, as this will dramatically increase your chances of detecting relevant disease.
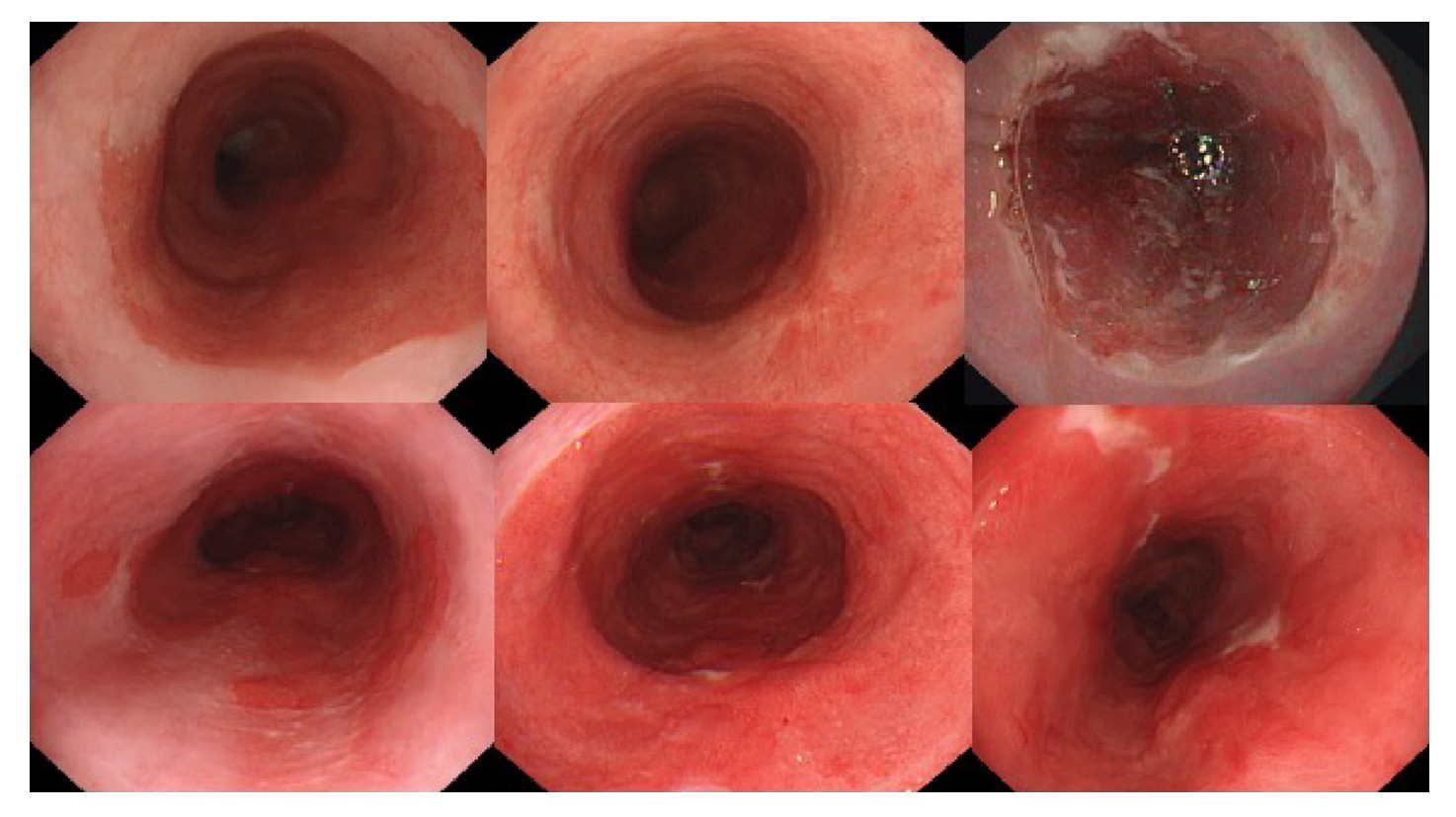
Use the waterjet of your endoscope to clean the Barrett segment—this generally takes 1–2 minutes. If you wonder whether you have cleaned the segment well enough, then it is generally not ‘just right’ yet. Switch to optical chromoscopy: if the oesophagus looks impeccably clean on narrow-band imaging (NBI), then your cleaning is optimal (figure 1).
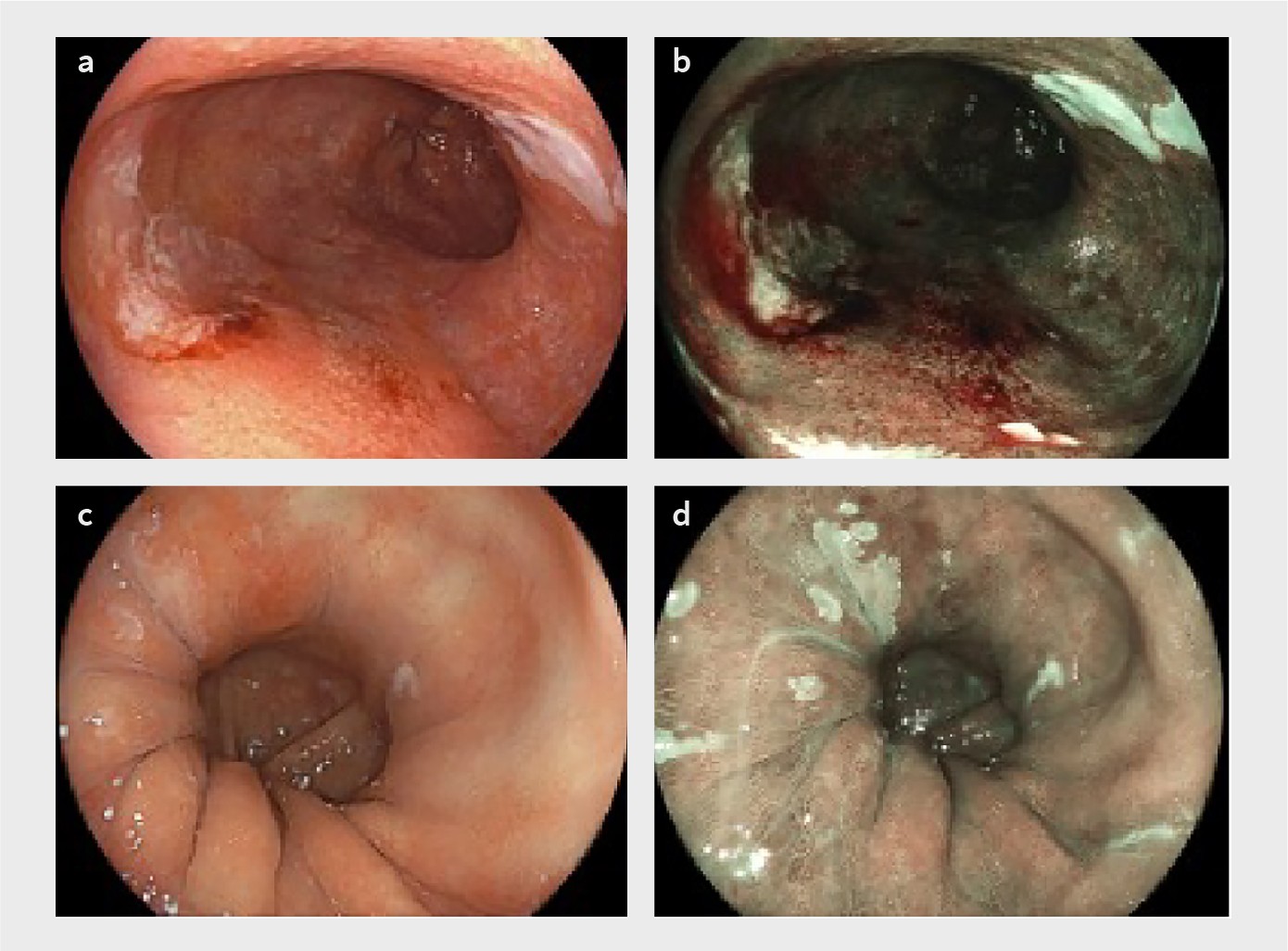
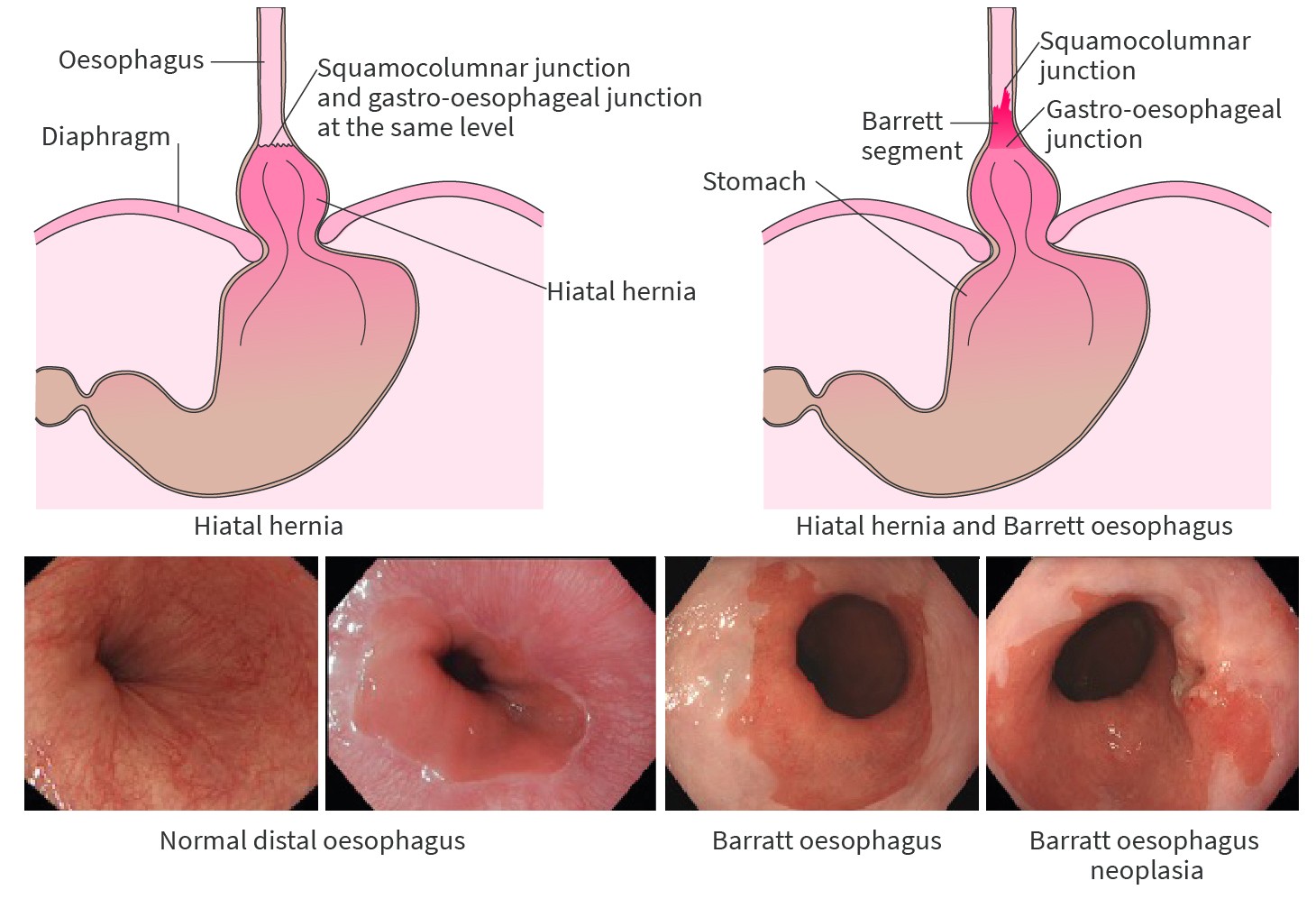
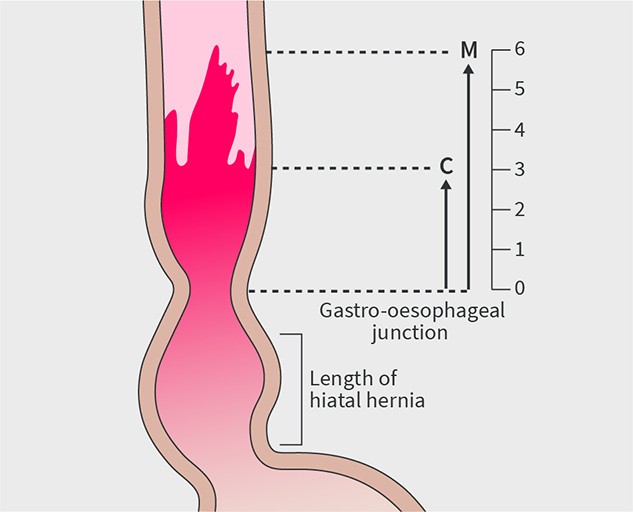
Subsequently, you should spend 3–5 minutes inspecting the segment using white light endoscopy. Switching back-and-forth with optical chromoscopy, which will create a different perspective, will help you to see more. Retroflex the endoscope to inspect the ‘danger zone’—the area where the Barrett segment transits into the hiatal hernia (figures 2 and 3). This is the area that has the highest risk of neoplasia and the highest risk of neoplasia being missed endoscopically. Look longer, biopsy less! After taking your first biopsy sample most of your imaging opportunities are lost.
Finally, know the face of Barrett oesophagus neoplasia (figure 2). Detecting early neoplasia is all about recognizing how early neoplasia actually looks. Excellent training modules are available for this at BEST Academia [www.best-academia.eu].
…a lesion that clearly looks neoplastic on endoscopy generally is neoplastic
You should not automatically be reassured if a biopsy sample taken from a visible abnormality in a Barrett oesophagus segment is diagnosed as non-dysplastic or inflammatory. In reality, this situation requires either endoscopic resection of the abnormality for optimal diagnosis or repeat endoscopy to document its regression, because a lesion that clearly looks neoplastic on endoscopy generally is neoplastic. Keep in mind that it is also not impossible for biopsy samples to be misplaced and that the histological assessment might not always be accurate. Communication with your pathologist on what you have biopsied is imperative for targeted biopsy samples. If your level of suspicion is high, please let your pathologist know. If you are uncertain whether your target lesion is inflammatory and your level of suspicion is low (e.g. when you have biopsied close to the squamocolumnar junction in the presence of a grade A or B reflux oesophagitis), please provide this information as well.
Optimal imaging allows you to make the right decision regarding resection versus ablation and it allows optimal delineation of lesions.
We prefer to use a diagnostic endoscope for most therapeutic work in patients with Barrett oesophagus. Optimal imaging allows you to make the right decision regarding resection versus ablation and it allows optimal delineation of lesions. As already mentioned, the availability of a waterjet is essential to ensure adequate cleaning of the Barrett oesophagus segment.
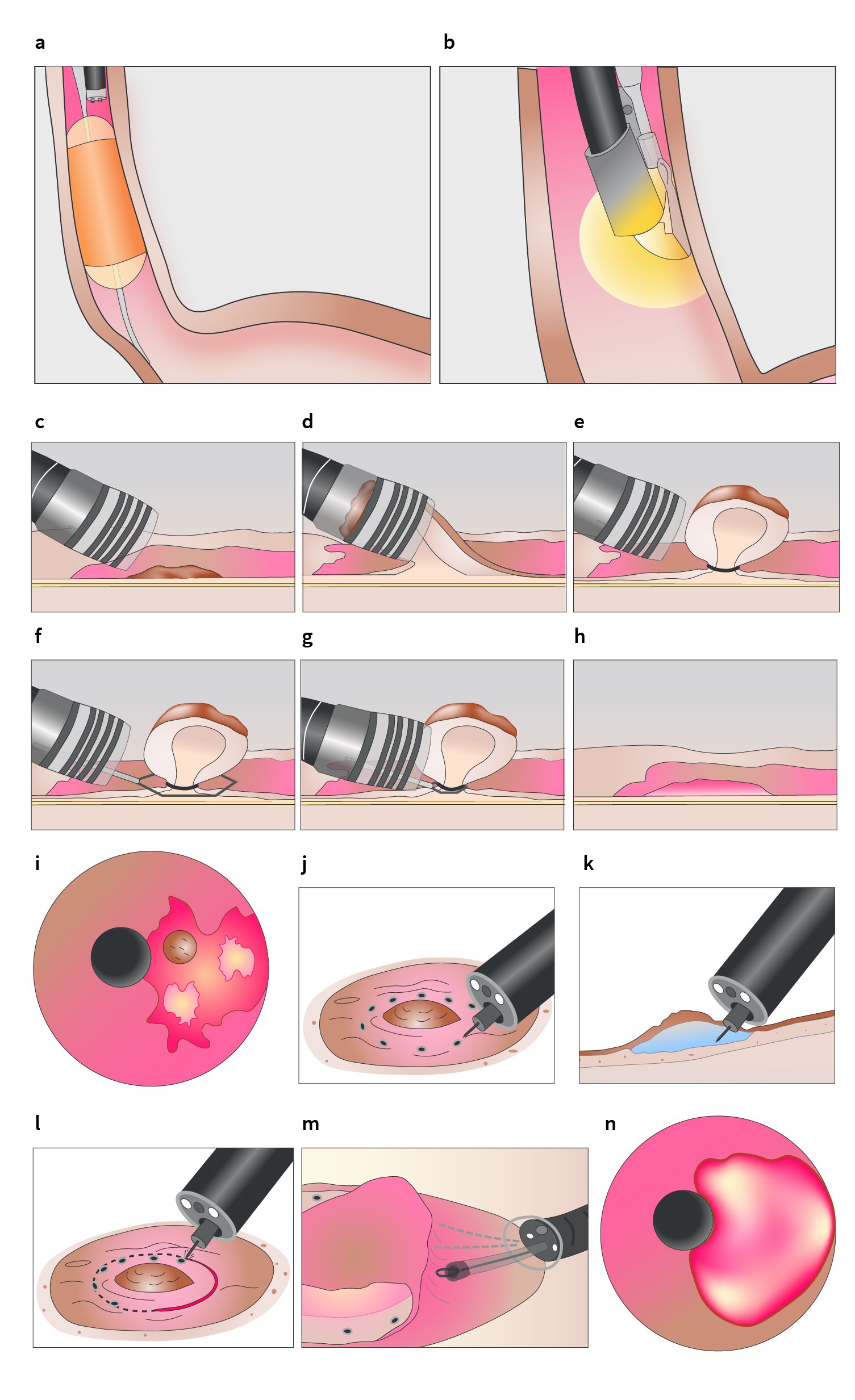
During endoscopic resection, deal with bleeding before proceeding with your endoscopic submucosal dissection (ESD) or piecemeal resection (figure 4). For piecemeal resections, any bleeding from prior resections must be adequately treated and the surface cleaned of blood and mucous. This also means that you should have emptied the stomach of fluids and blood before you embark on your next resection. If this stage is omitted, the stomach contents will reflux into the oesophagus while you are performing your next resection and may complicate matters if your next resection results in a bleed.
Be meticulously systematic at this stage: the time spent on optimizing imaging and the circumstances prior to resection always pays off in the end. In order to retrieve your specimens from the stomach at the end of the procedure you will need to empty the stomach of fluids anyway, so why not do this during the procedure to improve safety and efficacy of your resections?
…ablation shouldn’t be used as an excuse for not having to do an endoscopic resection
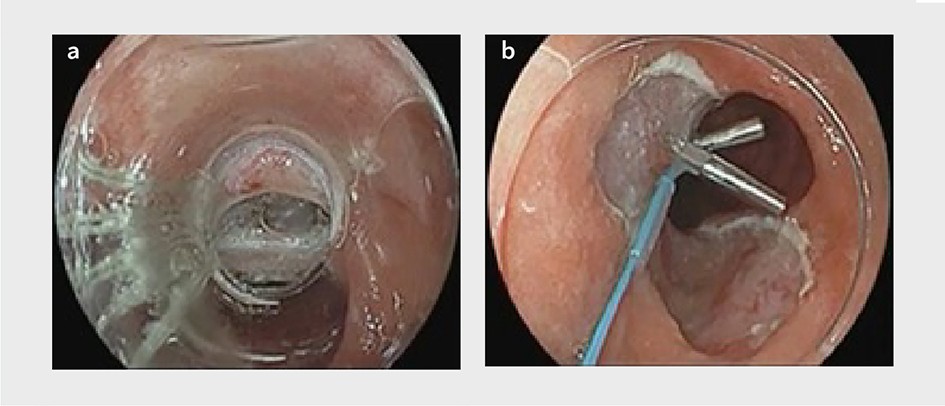
The most common reason for neoplastic progression under ablation therapy is that at one of the ablation sessions a lesion has been ablated whereas it should have been resected (figure 4a and b). If you don’t recognize visible lesions prior to ablation therapy, you run the risk of significantly delaying their diagnosis. If these lesions are ablated instead of resected, the first opportunity to pick them up is when the patient returns for the 3-month follow-up endoscopy.
For this reason, the endoscopist performing ablation therapy in patients with Barrett oesophagus should be able to switch gears to endoscopic resection. Indeed, ablation shouldn’t be used as an excuse for not having to do an endoscopic resection. If you have not yet mastered the skills necessary to perform endoscopic resection then it is vital that you do so before embarking on endoscopic ablation in Barrett oesophagus.
If the Barrett oesophagus segment is inflamed then ablation will not be effective...
Ablation sessions are generally scheduled at 3-month intervals. In the majority of cases, this time is sufficient for the oesophagus to have healed after the prior ablation, provided the patient has received adequate acid suppression therapy. However, there is a subgroup of patients with delayed healing who will have a swollen, oedematous, Barrett oesophagus segment with exudates after the 3-month interval (figure 5). Please do not proceed with ablation in these cases. Prolong the interval between the ablation sessions instead and check the adequacy of your acid suppressant therapy.
If the Barrett oesophagus segment is inflamed then ablation will not be effective, given the thickness of the epithelium and the likely inadequate acid suppression. In addition, you run the risk of prolonging the period in which you will not be able to adequately inspect the segment for neoplastic progression. Continuing multiple 3-monthly cycles of ablation followed by poor healing will not allow you to detect neoplastic progression, especially when you have overlooked a visible lesion at the initial ablation.
Marking the target area … before you begin the resection itself means you will have a roadmap to guide you … and prevent you from getting lost…
It is important to have a ‘pre-resection plan’ prior to embarking on the actual resection. The outer margins of your neoplastic target area may not be sufficiently visible once the multiband mucosectomy (MBM) kit has been assembled. In addition, resections and bleeding may hamper visualisation during piecemeal procedures. Marking the target area with electrocautery markings before you begin the resection itself means you will have a roadmap to guide you through the remaining procedure and prevent you from getting lost in less-than-optimal imaging circumstances present during the MBM procedure (figure 4c–h).
Given the importance of marking, we generally use a diagnostic endoscope with a small distant attachment cap for this purpose. The cap allows us to stabilize the endoscope tip onto the mucosa during the marking. The use of optical chromoscopy and a near-focus mode (with Olympus endoscopes) or zoom function (with Fujifilm or Pentax endoscopes) enables both the detection of the demarcation line and the controlled positioning of the electrocoagulation markers with the tip of the snare (for MBM procedures) or the ESD knife (figure 4i–n).
...there is a subgroup of lesions that should not be resected by MBM because of the likelihood that there is deep submucosal invasion and/or a large intraluminal extent of the lesion
Most of the early neoplastic lesions in Barrett oesophagus can be effectively removed by MBM. However, there is a subgroup of lesions that should not be resected by MBM because of the likelihood that there is deep submucosal invasion and/or a large intraluminal extent of the lesion. It is vital that endoscopists refrain from attempting MBM or endoscopic mucosal resection (EMR) for such lesions since it bound for failure and complications, and it compromises any subsequent endoscopic resection being performed by more experienced endoscopists. In case of doubt, obtain images and consult a more experienced colleague for their advice.
…therapy for Barrett oesophagus needs to be centralized
All guidelines state that adequate training and a yearly case volume of at least 10 new patients with high-grade dysplasia or early cancer are needed for an endoscopist to be allowed to embark on treating new patients. To ensure these guidelines are met, therapy for Barrett oesophagus needs to be centralized. For instance, in the Netherlands all endoscopic ablations and resections performed in patients with Barrett oesophagus are centralized to eight centres—not one ablation or resection is performed outside these centres. As a result, the outcomes are clearly superior to those in geographic areas where treatment is not centralized.2,3
After MBM procedures, where the resection is ‘blind’ and less controlled than in ESD, the bleeding source may be more difficult to determine
Most ESD bleeds can be managed by directing therapy to the vessel that has been accidentally cut. Coagulation forceps will do the job here. After MBM procedures, where the resection is ‘blind’ and less controlled than in ESD, the bleeding source may be more difficult to determine.
There are a few general rules when managing MBM bleeds. Do not remove the MBM cap unless it is absolutely necessary, because most bleeds can be treated by touching the bleeding site with the tip of the snare. Isolate the bleeding site by interrogating the resection site through the cap—you can use the edge or lateral side of the cap to compress the bleeding side. Finally, once you have identified the bleeding side only apply very gentle pressure with the tip of the snare.
Remember that you have already resected the mucosa and a significant part of the submucosa and that applying too much pressure may lead to a perforation. A careful snare tip coagulation generally suffices, but if the bleeding continues despite two or three applications you need to switch gears to coagulation forceps.
This switching will require you to release the remaining rubber bands in the stomach to allow passage of the forceps.
Most bleeds can be adequately managed with just snare-tip coagulation. After the bleeding is under control, remember to clean the surface area and to evacuate all fluids and blood from the stomach before you embark on your next resection.
…avoid spending too much time ‘thinking’ without actually ‘doing’
Perforations after MBM in patients with Barrett’s oesophagus are, thankfully, rare, occurring in 0.8–0.9% of cases (figure 6). They are never life threatening unless you make them.
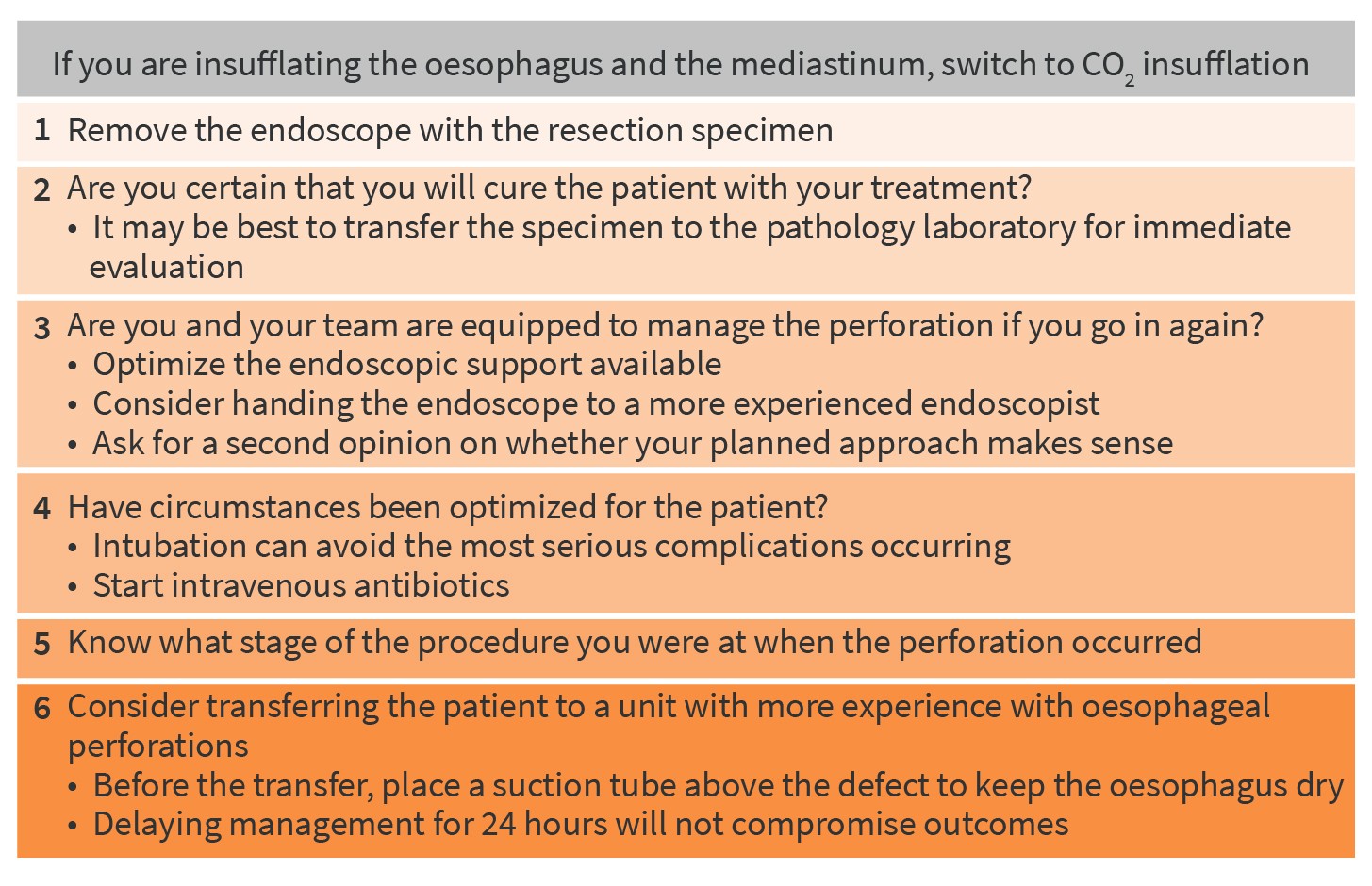
If a post-resection perforation occurs while you are insufflating the oesophagus and the mediastinum, avoid spending too much time ‘thinking’ without actually ‘doing’. Such delayed action can cause pneumothorax and pneumediastinum, and that indeed can be life threatening. If you are not already using CO2 insufflation, then immediately switch to it. If you do not have CO2 insufflation available, then you should not be performing therapeutic endoscopies.
In these circumstances, there are several steps that should be followed (figure 7). First, you should remove the endoscope with the resection specimen. Second, question how certain you are that you will cure the patient with your treatment. If you think you may have resected a deep submucosal cancer, it may be best to transfer the specimen to the pathology laboratory for immediate evaluation. Taking this action can make you switch gears to the surgery that’s inevitably required to manage both the neoplastic condition and the perforation, instead of managing the acute complication first and having to send the patient to surgery anyway. In the acute setting, your surgical colleague has the best chance of performing an optimal oesophagectomy. If you have to knock on their door after a week because your pathology specimen has come back with an irradically (incompletely) resected submucosal cancer while the patient is still in the intensive care unit for treatment of the mediastinitis you will rightfully be criticized for having compromised the chances of effective surgical treatment of both problems.
Third, decide whether you and your nursing team are equipped to manage the perforation if you go in again. It may be wise to optimize the endoscopic support available and/or to hand the endoscope to a more experienced endoscopist. For significant complications, we always insist on having an additional pair of endoscopist’s eyes in the room to reveal any blind spots we may have. Revealing blind spots is not necessarily related to experience, so even if you are the most experienced endoscopist in your unit we suggest you should always be prepared to ask somebody else in the room to check if your planned approach to tackle the problem makes sense.
Fourth, check whether you have optimized circumstances for the patient. In most cases it may be wise to intubate the patient to avoid the most serious complications occurring (i.e. pneumothorax and pneumomediastinum). Start intravenous antibiotics.
Fifth, know what stage of the procedure you were at when the perforation occurred. Had you finished your endoscopic resection or were you halfway through your piecemeal resection? We generally complete the endoscopic resection before attempting to close the defect with clips or loops. In selected cases, we will bridge the defect with a stent (in case of a pre-existing stenosis, which will keep the stent in place) or with a vacuum sponge.
Finally, consider transferring the patient to a unit that has more experience in the endoscopic management of oesophageal perforations. If this is the best option, place a suction tube above the defect to keep the oesophagus dry and then transfer the patient. Be confident that delaying management for 24 hours will not compromise outcomes, whereas trying to do something out of your league could make things significantly worse.
-
About the authors
-
Your Barrett oesophagus neoplasia briefing
UEG Week
- ‘Barrett neoplasia: EMR or ESD?’ presentation in the ‘Therapy update: Upper GI interventional endoscopy’ session at UEG Week Virtual 2021.
- ‘Barrett’s Oesophagus: What’s hot?’ session at UEG Week Virtual 2021.
- ‘Endoscopic diagnosis of oesophageal neoplasia’ session at UEG Week Virtual 2021.
- ‘Barrett’s oesophagus’ session at UEG Week Virtual 2020.
- ‘Barrett's oesophagus’ presentation in the ‘Screening – surveillance: Premalignant conditions’ session at UEG Week Virtual 2020.
- ‘Mistakes in the endoscopic diagnosis and management of Barrett's oesophagus’ presentation in the ‘Mistakes in…’ session at UEG Week Virtual 2020.
- ‘Barrett’s oesophagus’ session at UEG Week 2019.
Mistakes in...
- Haidry RJ and Magee C. Mistakes in the endoscopic diagnosis and management of Barrett’s oesophagus and how to avoid them. UEG Education 2018; 2018: 12–14.
Standards and Guidelines
- de Pietro M, et al. Revised British Society of Gastroenterology recommendation on the diagnosis and management of Barrett’s oesophagus with low-grade dysplasia. Gut 2018; 67: 392–393.
- Weusten BLAM, et al. Endoscopic management of Barrett’s esophagus: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2017; 49: 191–198.
- Bisschops R, et al. Performance measures for upper gastrointestinal endoscopy: A European Society of Gastrointestinal Endoscopy quality improvement initiative. United European Gastroenterology Journal 2016; 4: 629–656.
Please log in with your myUEG account to post comments.